
INTRODUCING
Dr. Mor Sendovsky Goldfeder
Ph.D. Student 2013
Rational Design and Structure-Based Investigations of a
Tyrosinase from Bacillus megaterium
The goal of this research was to structurally investigate tyrosinase from Bacillus megaterium through the determination of its crystal structure for the elucidation of its structure-function properties.
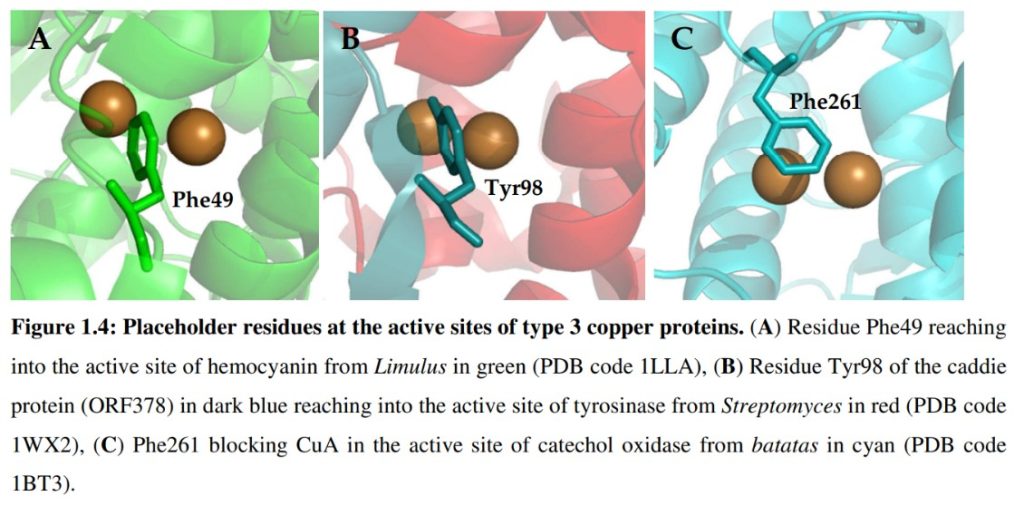
Tyrosinase from the soil bacterium Bacillus megaterium (TyrBm) was previously isolated and characterized in our lab. This work initiated with finding conditions for crystallization of TyrBm. Crystals were obtained and the enzyme was shown to be active in crystal. Structures were determined to a resolution of 2.0Å. The active site copper ions, coordinated by six conserved histidine residues, varied in occupancy and in position.
Second-shell residues surrounding the active site were investigated for their influence on activity and selectivity. Residues R209 and V218 were shown to play a role in substrate orientation, due to their flexibility and proximity to the di-copper center.
Investigating copper accumulation in TyrBm, it was found that copper concentration has a more significant effect on the diphenolase activity. Using rational design, five variants were identified as having an impact on copper uptake. A mechanism for copper accumulation by the enzyme was proposed.
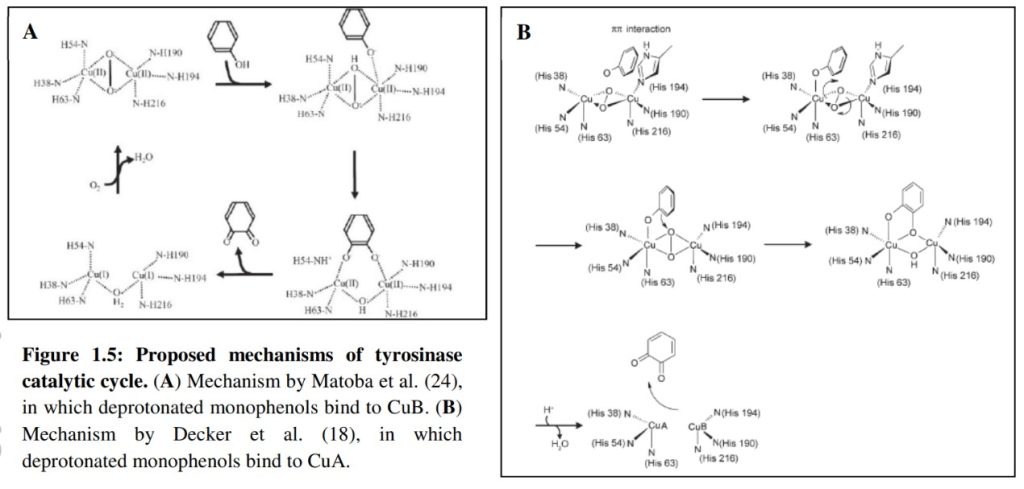
For the first time, structures of a tyrosinase with substrates in the active site were obtained by replacing copper ions with zinc. Structures with L-tyrosine and L-Dopa show that both substrates bind identically at the active site, towards CuA, as opposed to the prevalent models in the literature. Furthermore, the same orientation was observed in a structure determined with p-tyrosol and copper ions. Altogether, the determined structures elucidate parts of the catalytic mechanism of tyrosinase and related proteins.
The effect of ionic liquids (ILs) and sodium dodecyl sulphate (SDS) on the activity of TyrBm was investigated. In the presence of two water miscible ILs the monophenolase/diphenolase activity ratio increased up to 5-fold. The addition of up to 50 mM SDS increased the activity 15-20 fold towards the non-native substrates phenol and catechol. A structure determined in the presence of an SDS molecule shows it affects residue R209, enabling less polar substrates to penetrate more efficiently into the enzyme catalytic pocket.
In summary, in this work, structural data was obtained for the wild-type enzyme and various mutants, thus providing significant new information about tyrosinases. The copper uptake mechanism was established; the role of second-shell residues and their influence on activity and selectivity was understood, as well as the effect of additives. This new information can be further used to engineer TyrBm for various biotechnological applications.
Read Ph.D Thesis

Mor Goldfeder with Ayelet Fishman receiving her PhD degree



